Chap. 4 electrolysis cell
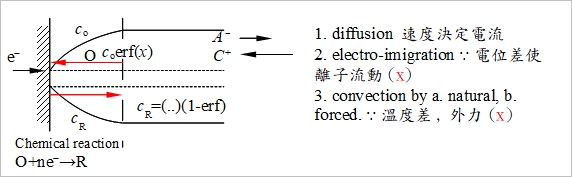
當E>>E₀
at x=0, E=E₀-(RT/nF)ln(cR/cₒ)|ₓ₌₀ cR/cₒ→0 as cR<<cₒ ⸫R→O
limiting current, iᴅₐ=-nFAcR*DR/XᴅR....(1), iᴅc=-nFAcₒ*Dₒ/Xᴅₒ...(2)
general case:
i= -nFAJ₀|ₓ₌₀=nFAJR|ₓ₌₀
Jₒ|ₓ₌₀= -Dₒ[cₒ*-cₒ(0,t)]/Xᴅₒ=-i/nFA → cₒ(0,t)=cₒ*-(i/nFA)(Xᴅₒ/Dₒ)....(3)
(2)代入(3), 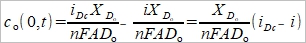 ....(5)
....(5)
同理, cR(0,t)=cR*+(i/nFA)(XᴅR/DR)....(4)
(1)代入(4), 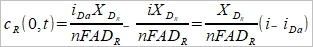 .….(6)
.….(6)
E=E°+(RT/nF)ln[cₒ(0,t)/cR(0,t)]....(4.16)
(5), (6)代入(4.16),
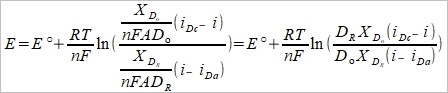 ⸪ Xᴅᵢ=(πDᵢt)½
⸪ Xᴅᵢ=(πDᵢt)½
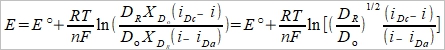
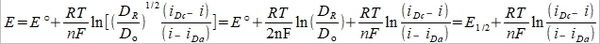
(7)
i.e. E½= E°+(RT/2nF)ln(DR/Dₒ)=E°-(RT/2nF)ln(Dₒ/DR)

實驗上把時間固定
-
dme: dropping mercury electrode
-
rotation disk → steady state electrode
applcation:
⸪t=constant, Xᴅₒ=(πDₒt)½=constant
iᴅc=δₒcₒ* ….(8)→ δₒ= nFADₒ/Xᴅₒ
iᴅₐ=-δRcR*....(9)
O+ne⁻→R, x: mole fraction of O cₒ*=xc* and cR*=(1-x)c*
(8), (9)代入(7)→ E=E½+(RT/nF)ln[(δₒxc*-i)/(i+δR(1-x)c*)]
可作i-E-x的圖, 如Fig. 4.12
-
當x固定, E-i圖
-
i=0, E-x圖: potentiometric titration curve, Fig. 4.13(b)
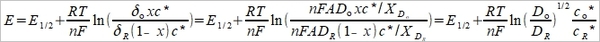
⸪ Xᴅᵢ=(πDᵢt)½, and E½=E°-(RT/2nF)ln(Dₒ/DR)
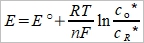 → Nerst eq., +號是因為O+ne⁻→R(還原電位)
→ Nerst eq., +號是因為O+ne⁻→R(還原電位)
-
E=constant, i-xdiagram
constant composition, iᴅc=-nFAcₒ*Dₒ/Xᴅₒ≈kct⁻½ Xᴅₒ=(πDₒt)½
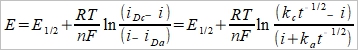 , → i-t-E diagram
, → i-t-E diagram
-
i=constant, chronopotentiometry
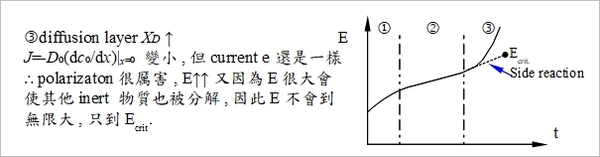
2. E=constant, 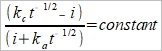 i-t圖
i-t圖
Dropping mercury electrode(d.m.e.)
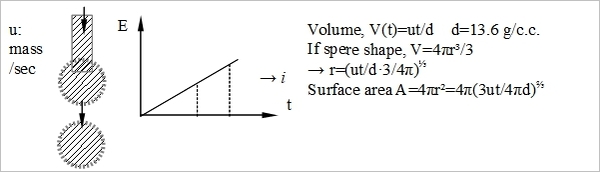
當V↑, Xᴅ↓ 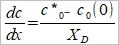 ↑ D₀dme≈7D₀/3
↑ D₀dme≈7D₀/3
iᴅ=nFAcₒ*Dₒ/Xᴅₒ=nFAcₒ*(Dₒ/πt)½=nF4π(3ut/4πd)⅔cₒ*(7Dₒ/3πt)½=706nDₒ½cₒ*u⅔t⅙



