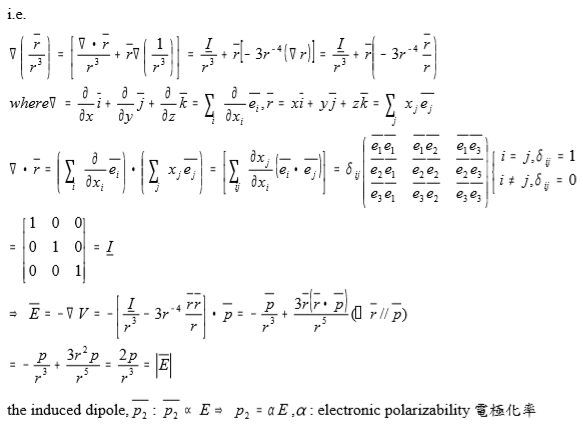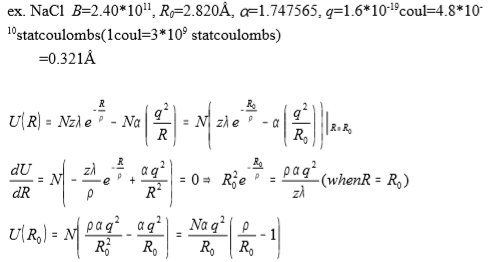Chap. 3 Crystal binding
Principle types of crystalline binding
-
crystal of inert gas(VIII A): van der Wals force; dipole-to-dipole interaction
-
ionic crystal: NaCl electrostatic force between ions(IA & VIIA)
-
covalent crystal: C, Si(IVA) quantum effect –spin repulsive or attractive by exchange force.
-
metallic crystal: Na, K(IA, B) charge attraction between positive and negative charges.
Crystal of inert gas
不考慮thermal vibration if –q分布中心在中心,則場效應=0
|
He |
Ne |
Ar |
Kr |
Xe |
|
hcp |
( fcc ) | |||
|
1s² |
1s² 2s² 2p⁶ |
(Ne) 3s² 3p⁶ |
(Ar) 3d¹⁰ 4s² 4p⁶ |
(Kr) 4d¹⁰ 5s² 5p⁶ |
if thermal vibration at 0K, zero-point energy = ½ℏω=½cu²=½mw²u²
u²1/2平均距離, u:位移, c:彈性係數 k
binding force in the inert gas crystal at instant t :
the interaction energy between two diploes, p₁, p₂, separated by R is given by
U(R)=(p₁∙p₂)/R³-3(p₁∙R)(p₂∙R)/R⁵ → U(R)=(p₁∙p₂)/R³-3(p₁∙p₂)/R³=-2(p₁∙p₂)/R³
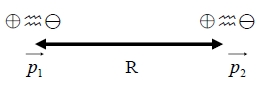
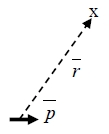
V=p∙R/R³, E=-V=-(p∙R/R³)=-p∙(R/R³)-(R/R³)∙ p
p=0, 與電荷密度有關∵r距離太遠
i.e.
(R/R³)=[∙R/R³+R(1/R³]=I/R³+R[-3R⁻⁴(R)]=I/R³+R[-3R⁻⁴(R/R)]
where =(∂∕∂x)i+(∂∕∂y)j+(∂∕∂z)k=∑ᵢ (∂∕∂xᵢ)eᵢ, r=xi+yj+zk=∑j xj ej ,
∙r=[∑ᵢ (∂∕∂xᵢ)eᵢ]∙[∑j xj ej]=[∑ᵢj (∂xj ∕∂xᵢ)(eᵢ∙ej )]=δᵢj =
=
=I, i.e. δᵢj=1 if i=j or δᵢj=0 if i≠j
E=-V=-[I/R³-3R⁻⁴(R∙R/R)]∙p=-p/r³+3R(R∙p/R⁵)=-p/R³+3R²p/R⁵=2p/R³=|E| ⸪ R// p
the induced dipole, p₂ : p₂ E→p₂=αE, : electronic polarizability電極化率
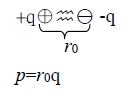
⸫ p₂=α(2p/R³)=2αqr₀/R³ and U(R)=-2(p₁∙p₂)/R³=(-2/R³)∙r₀q∙(2αqr₀/R³)
=-4α(r₀q)²/R⁶=-A/R⁶ → F=-U=-dU/dR 1/R⁷
Quantum derivation of van der Waals force
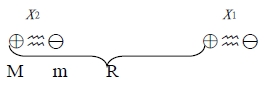
total energy, H=(p₁²/2m+½cx₁²)+(p₂²/2m+½cx₂²)+H₁, H1: 靜電位能
ex. H原子, Eₙ=(n+½)ℏω
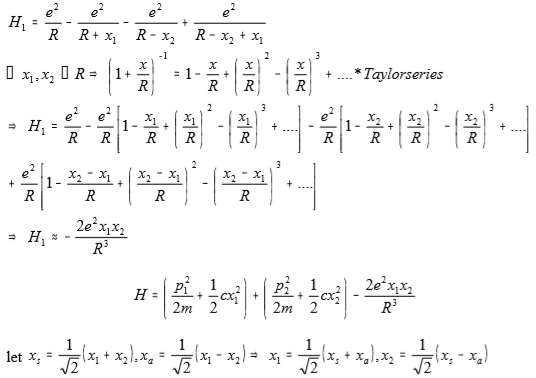
H₁=e²/R-e²/(r+x₁)-e²/(R-x₂)+e²/(R-x₂+x₁)
⸪ x₁, x₂<<R → (1+R/r)⁻¹=1-x/R+(x/R)²-(x/R)³+... Taylor series
H₁=e²/R-e²/R[1-x₁/R+(x₁/R)²-(x₁/R)³+...]-e²/R[1-x₂/R+(x₂/R)²-(x₂/R)³+...]+e²/r[1-(x₂-x₁)/R+(x₂-x₁)²/R²-(x₂-x₁)³/R³+...] → H₁≈-2e²x₁x₂/R³
H=(p₁²/2m+½cx₁²)+(p₂²/2m+½cx₂²)-2e²x₁x₂/R³
let xₛ=1/√2(x₂+x₁), xₐ=1/√2(x₁-x₂) → x₁=1/√2(xₛ+xₐ), x₂=1/√2(xₛ-xₐ)
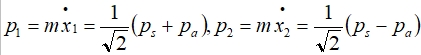
H=(p₁²/2m+½cx₁²)+(p₂²/2m+½cx₂²)-2ex₁x₂/R³
={[1/√2(pₛ+pₐ)²/2m]+½c[1/√2(xₛ+xₐ)]²}+{[1/√2(pₛ-pₐ)²/2m]+½c[1/√2(xₛ-xₐ)]²}-2e²[1/√2(xₛ+xₐ)][1/√2(xₛ-xₐ)]/R³
=[½(pₛ²+2pₛpₐ+pₐ²+pₛ²-2pₛpₐ+pₐ²)]/2m+c/2[½(xₛ²+2xₛxₐ+xₐ²)+½(xₛ²-2xₛxₐ+xₐ²)]-e²(xₛ²-xₐ²)/R³=pₛ²/2m+pₐ²/2m+½cxₛ²+½cxₐ²-e²(xₛ²-xₐ²)/R³
=[pₛ²/2m+½(c-2e²/R³)xₛ²]+[pₐ²/2m+½(c+2e²/R³)xₐ²]
∵為Simple Harmonic motion Eₙ=(n+½)ℏω₀
if H=H0+H1 H=E (H0+H1)=E H0+H1=E
*The zero point energy(n=0) of the system
½ ħ(ωs+ωa)—with dipole-to-dipole interaction, H0+H1(Ep.p.+ E0-0)
½ ħ(ω0+ω0)—without D-D interaction(E0-0)
∆U=½ ħ(ωs+ωa)-½ ħ(ω0+ω0)
ω0=(c/m)1/2, ωs=[(c-2e²/R³)/m]1/2=(c/m)1/2[1-½(2e²/cR³)-⅛(2e²/cR³)²+...]
ωa=[(c+2e²/R³)/m]1/2=(c/m)1/2[1+½(2e²/cR³)-⅛(2e²/cR³)²+...]
→ ∆U=½ ħ(ωs+ωa)-½ ħ(ω0+ω0)≈ ½ ħ[ω0+ω0-¼ω0 (2e²/cR³)²]-½ ħ(ω0+ω0)
=-ℏω₀/8(2e²/cR³)²
Repulsive interaction—due to the overlapping of electron orbit[Pauli exclusion principle]
Empirical form B/R¹²
the total potential energy of two inert gas atoms at separation R is
U(R)=B/R¹²-A/R⁶=4ε[(σ/R)¹²-(σ/R)⁶]
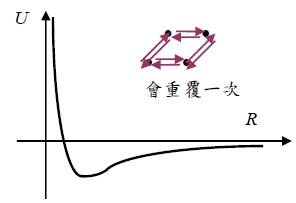
Equilibrium lattice constant
Uₜₒₜₐₗ=½∑ᵢ ∑j Uij=½∑j (∑ᵢUij)=(N/2)∑jUij
now Uij=4ε[(σ/Rij)¹²-(σ/Rij)⁶ ] let Rij=Pij R → Uij=4ε[(1/Pij)¹²(σ/R)¹²-(1/Pij)⁶(σ/R)⁶]
Uₜₒₜₐₗ=½N4ε[∑j(1/Pij)¹²(σ/Rij)¹²-∑j(1/Pij)⁶(σ/Rij)⁶]
For inert gas crystal, in addition to He.
-
nearest neighbors: 12, Pij=1 and R=a/√2
-
second nearest neighbors: 6, Pij=√2 and R’=√2R=a
-
third nearest neighbors: 12, Pij=2 and R”=2R=√2a….
∑j(1/Pij)¹²=12∙1⁻¹²+6∙√2⁻¹²+12∙2⁻¹²+...=12.1388
∑j(1/Pij)⁶=12∙1⁻⁶+6∙√2⁻⁶+12∙2⁻⁶+...=14.45392
Uₜₒₜₐₗ=2Nε[12.1388(σ/R)¹²-14.45392(σ/R)⁶]
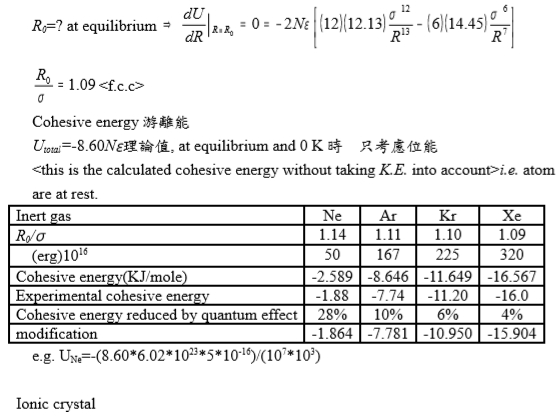
R0=? at equilibrium dU/dR|R=R₀=0=-2Nε[12.13(12σ¹² /R¹³)-14.45(6σ⁶/R⁷)]
R0/σ=1.09
Cohesive energy游離能
Utotal=-8.60N理論值, at equilibrium and 0 K時, 只考慮位能
K.E. into account>i.e. atom are at rest.
|
Inert gas |
Ne |
Ar |
Kr |
Xe |
|
R0/ |
1.14 |
1.11 |
1.10 |
1.09 |
|
ε(erg)1016 |
50 |
167 |
225 |
320 |
|
Cohesive energy(KJ/mole) |
-2.589 |
-8.646 |
-11.649 |
-16.567 |
|
Experimental cohesive energy |
-1.88 |
-7.74 |
-11.20 |
-16.0 |
|
Cohesive energy reduced by quantum effect |
28% |
10% |
6% |
4% |
|
modification |
-1.864 |
-7.781 |
-10.950 |
-15.904 |
e.g. UNe=-(8.606.021023510-16)/(107103)
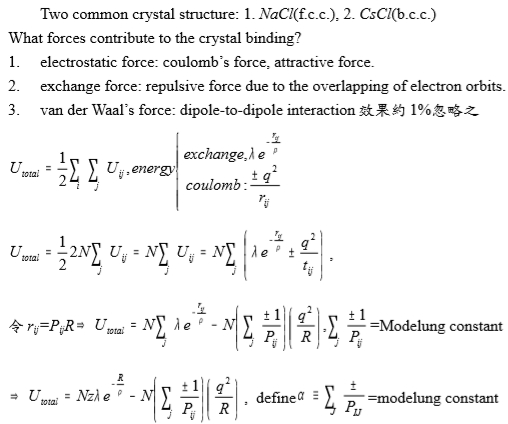
Ionic crystal
Two common crystal structure: 1. NaCl(f.c.c.), 2. CsCl(b.c.c.)
What forces contribute to the crystal binding?
-
electrostatic force: coulomb’s force, attractive force.
-
exchange force: repulsive force due to the overlapping of electron orbits.
-
van der Waal’s force: dipole-to-dipole interaction效果約1%忽略之
Uₜₒₜₐₗ=½∑ᵢ ∑j Uij, energy includes(i)coulomb: ±q²/rij, (ii)exchange: λe-rij/ρ
Uₜₒₜₐₗ=½∙2N∑jUij=N∑jUij=N∑j(λe-rij/ρ±q²/rij)
令rij=PijR, Uₜₒₜₐₗ=N∑jλe-rij/ρ-N∑j(±1/Pij)(q²/R), ∑j(±1/Pij)=Modelung constant
Uₜₒₜₐₗ=Nzλe-R/ρ-N∑j(±1/Pij)(q²/R) , define α≡∑j(±1/Pij)=modelung constant
NaCl crystal(f.c.c.): pick Cl- as a reference ion
Nearest ion: 6Na+, R0
2nd nearest ions: 12 Cl-, √2R0
3rd nearest ion: 8 Na+, √3R0
α=[6∙1⁻¹+12∙√2⁻¹²+8∙√3⁻¹+...]=1.747565
CsCl crystal(b.c.c.) : pick Cs+- as a reference ion
Nearest ion: 8Cl-, R0=√3a/2
2nd nearest ions: 6Cs+, R=a=2R0/√3
3rd nearest ion: … α=1.762675
Uₜₒₜₐₗ=Nzλe-R/ρ-Nα(q²/R)
determination of , : characteristic force range
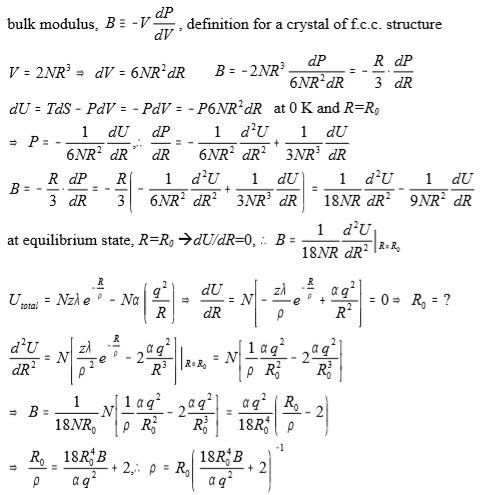
bulk modulus, B≡-VdP/dV, definition for a crystal of f.c.c. structure
V=2NR³ → dV=6NR²dR B=-2NR³dP/(6NR²dR)=-(R/3)(dP/dR)
dU=TdS-PdV=-PdV=-P6NR²dR at 0 K and R=R0
P=-(1/6NR²)dU/dR, ⸫ dP/dR=-(1/6NR²)d²U/dR²+(1/3NR³)dU/dR
B=-(R/3)(dP/dR)=-(R/3)[-(1/6NR²)d²U/dR²+(1/3NR³)dU/dR]
=(1/18NR)d²U/dR²+(1/9NR²)dU/dR]
at equilibrium state, R=R0 dU/dR=0, ⸫ B=(1/18NR)d²U/dR²|R=R₀
Uₜₒₜₐₗ=Nzλe-R/ρ-Nα(q²/R) → dU/dR=N[-zλe-R/ρ/ρ+αq²/R²]=0, R0²=ραq²/(zλe-R/ρ)
d²U/dR²=N[zλe-R/ρ/ρ²-2αq²/R³]|R=R₀=N[αq²/ρR₀²-2αq²/R₀³]
→ B=(1/18NR₀)∙N[αq²/ρR₀²-2αq²/R₀³]=αq²/18R₀⁴(R₀/ρ-2)
→ R₀/ρ=18R₀⁴B/αq²+2, ⸫ ρ=R₀(18R₀⁴B/αq²+2)⁻¹
ex. NaCl B=2.401011, R0=2.820Å, =1.747565, q=1.610-19coul=4.810-10 statcoulombs(1coul=3109 statcoulombs)
→ =0.321Å
U(R)=Nzλe-R/ρ-Nα(q²/R)=N[zλe-R₀/ρ-α(q²/R₀)]|R=R₀,
dU/dR=N[-zλe-R/ρ/ρ+αq²/R²]=0, R0²e-R/ρ=ραq²/(zλ) when R=R0
U(R0)=N[αq²ρ/R₀²-αq²/R₀]=Nαq²/R₀[ρ/R₀-1]