Chapter 5 Auxiliary Function
dU=TdS- PdV, U=U(S,V); dS=dU/T+(P/T)dV, S=S(U,V) 操作變數不方便,改成方便操作的壓力、體積、溫度的變數,需用何種函數?
通常量測上用壓力和溫度,理論推導用溫度與體積
Energy function def: A≡U-TS, H≡U+PV, G≡H-TS 目的:將2nd law轉變形式來看
微分式
dH=dU+PdV+VdP=TdS-PdV+PdV+VdP=TdS+VdP, H(S,P)
dA=dU-TdS-SdT=TdS-PdV-TdS-SdT=-SdT-PdV, A(T,V)
dG=dH-TdS-SdT=TdS+VdP-TdS-SdT=-SdT+VdP, G(T,P)
利用4個能量函數的微分式座運算處理:
dU=TdS- PdV, dH=TdS+VdP, dA=-SdT-PdV, dG=-SdT+VdP
H, Enthalpy H≡U+PV
等壓過程, dHₚ=TdS+VdP=TdSₚ=δQₚ
state 1 → state 2 ∆H=H₂-H₁=(U₂-U₁)+(P₂V₂-P₁V₁),
at constant P, ∆Hₚ=∆U+P∆V=∆Qₚ-∆W+P∆V →∆Hₚ=∆Qₚ, ∆H=進入或離開的熱(焓)
A, Helmholtz free energy A≡U-TS
state 1 → state 2 ∆A=A₂-A₁=(U₂-U₁)-(T₂S₂-T₁S₁),
at const T, ∆Aᴛ=(U₂-U₁)-T(S₂-S₁)=∆U-T∆S=∆Q-∆W-T∆S 因此, ∆Aᴛ+∆W=∆Q-T∆S
⸪ ∆Q≤∆Qᵣₑᵥ=T∆S → ∆Q-T∆S≤0 ⸫∆Aᴛ+∆W≤0 → ∆Aᴛ≤-∆W
i.e. ∆A+T∆Sₚᵣₒᵈ=-∆W → dA+TdSᵢᵣᵣₑᵥ=-δW
if constant V, δW=PdV=0 ⸫ dAᴛ,ᴠ+TdSᵢᵣᵣₑᵥ=0 under constant T and V,
dAᴛ,ᴠ= -TdSᵢᵣᵣₑᵥ≤0 (⸪2nd law: dSᵢᵣᵣₑᵥ≥0) A↓, Aᴛ,ᴠ= Aₘᵢₙ in equil.
Ex. 在固定溫度和體積的固體昇華成氣體,看能量與氣體粒子的關係
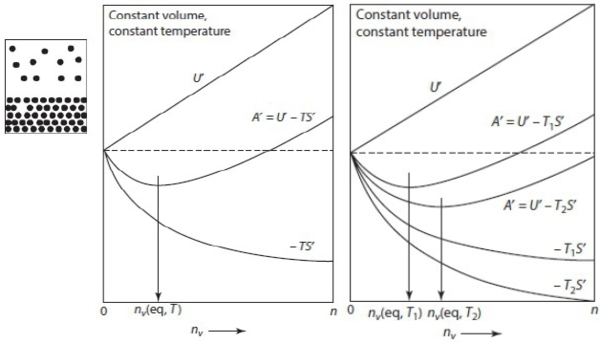
If P<Pₑզ → nᵥ< nᵥ,ₑզ
∆S熱儲=-Q/T...(1), ∆Sgas=+(Q/T)+∆Sₚᵣₒᵈ...(2), (1)+(2) ∆Stotal=∆Sₚᵣₒᵈ=∆Sᵢᵣᵣₑᵥ
for gas at constant T and V, ∆Aᴛ,ᴠ+T∆Sₚᵣₒᵈ=0 → ∆Aᴛ,ᴠ=-T∆Sₚᵣₒᵈ → ∆Aᴛ,ᴠ≤0
G, Gibbs free energy G≡H-TS
state 1 → state 2 ∆G=G₂-G₁=(H₂-H₁)-(T₂S₂-T₁S₁)=(U₂-U₁)+(P₂V₂-P₁V₁)-(T₂S₂-T₁S₁),
at const T and P, ∆Gᴛ,ᴩ=(U₂-U₁)+P(V₂-V₁)-T(S₂-S₁)=∆U+P∆V-T∆S=∆Q-∆W+P∆V-T∆S i.e.∆W=P∆V+∆W → ∆Gᴛ,ᴩ=∆Q-∆W-T∆S
⸪ ∆Q≤∆Qᵣₑᵥ=T∆S → ∆Q-T∆S≤0 ⸫∆Gᴛ,ᴩ+∆W≤0 → ∆Gᴛ,ᴩ≤-∆W
i.e. ∆Gᴛ,ᴩ+T∆Sₚᵣₒᵈ=-∆W if no other work, ∆W=0 ⸫ ∆Gᴛ,ᴩ+T∆Sₚᵣₒᵈ=0,
∆Gᴛ,ᴩ= -T∆Sᵢᵣᵣₑᵥ≤0 (⸪2nd law: ∆Sᵢᵣᵣₑᵥ≥0) G↓,
if ∆Gᴛ,ᴩ<0, span="">state 1 → state 2自然發生, 而且Gᴛ,ᴩ= Gₘᵢₙ at equilibrium point.
Summary: 如何判斷變化是否發生和平衡的條件
2nd law: dSᴜ,ᴠ≥0, 達平衡時Sᴜ,ᴠ=Sₘₐₓ 而且dSᴜ,ᴠ=0
dUS,ᴠ≤0, US,ᴠ=Uₘᵢₙ; dHS,ᴩ≤0, HS,ᴩ=Hₘᵢₙ; dAᴛ,ᴠ≤0, Aᴛ,ᴠ=Aₘᵢₙ; dGᴛ,ᴘ≤0, Gᴛ,ᴘ=Gₘᵢₙ
將第二定律entropy增加的條件轉換成能量下降的條件,方便操作!
能量函數定義: A≡U-TS, H≡U+PV, G≡H-TS 須記熟
微分式
dU=TdS- PdV, U(S,V)
dH=TdS+VdP, H(S,P)
dA=-SdT-PdV, A(T,V)
dG=-SdT+VdP, G(T,P)
Section 6&7 chemical work(potential)成分變化 G=G(T,P,ni,nj,…)
dG=(∂G∕∂T)ᴘ,nᵢ,...dT+(∂G∕∂P)ᴛ,nᵢ,...dP+(∂G∕∂nᵢ)ᴛ,ᴘ,nj,...dnᵢ+.. 若組成物莫耳數保持一定 dG=(∂G∕∂T)ᴘdT+(∂G∕∂P)ᴛdP
def: (∂G∕∂nᵢ)ᴛ,ᴘ,nj,...=μᵢ化學勢 dG=-SdT+VdP+Σμᵢnᵢ ,
i.e. (∂G∕∂nᵢ)ᴛ,ᴘ,nj,...=(∂A∕∂nᵢ)ᴛ,ᴘ,nj,...=(∂H∕∂nᵢ)ᴛ,ᴘ,nj,...=(∂U∕∂nᵢ)ᴛ,ᴘ,nj,...
熱力學的數學推導(係數關係)
dU=TdS- PdV=(∂U∕∂S)ᵥdS+(∂U∕∂V)ₛdV, T=(∂U∕∂S)ᵥ=(∂H∕∂S)ₚ
dH=TdS+VdP=(∂H∕∂S)ₚdS+(∂H∕∂P)ₛdP, S=-(∂A∕∂T)ᵥ=-(∂G∕∂T)ₚ
dA=-SdT-PdV=(∂A∕∂T)ᵥdT+(∂A∕∂V)ᴛdV, P=-(∂U∕∂V)ₛ=-(∂A∕∂V)ᴛ
dG=-SdT+VdP=(∂G∕∂T)ₚdT+(∂G∕∂P)ᴛdP, V=(∂H∕∂P)ₛ=(∂G∕∂P)ᴛ
Maxwell relation :
if Z=Z(x,y), dZ=(∂Z∕∂x)ydx+(∂Z∕∂y)xdy=Ldx+Mdy and (∂L∕∂y)x=(∂M∕∂x)y
∴(∂T∕∂V)ₛ=-(∂P∕∂S)ᵥ, (∂T∕∂P)ₛ=(∂V∕∂S)ₚ, (∂S∕∂V)ᴛ=(∂P∕∂T)ᵥ, -(∂S∕∂P)ᴛ=(∂G∕∂T)ₚ

Derived equations
Ex-1. ideal gas: U=U(T), (∂U∕∂V)ᴛ=0, (∂U∕∂P)ᴛ=0
pf: dU=TdS- PdV
(∂U∕∂V)ᴛ=T(∂S∕∂V)ᴛ-P=T(∂P∕∂T)ᵥ-P=T(R/V)-P=0,
(∂U∕∂P)ᴛ=T(∂S∕∂P)ᴛ-P(∂V∕∂P)ᴛ=-T(∂V∕∂T)ₚ-P(-RT/P²)=-T(R/P)+RT/P=0
or (∂U∕∂S)ᴛ=T-P(∂V∕∂S)ᴛ=T-P(∂T∕∂P)ᵥ=T-P (R/V)=0 所以U為T的函數, 與壓力,體積, S均無關
Ex-2. ideal gas: H=H(T), (∂H∕∂V)ᴛ=0, (∂H∕∂P)ᴛ=0
pf: dH=TdS+VdP
(∂H∕∂V)ᴛ=T(∂S∕∂V)ᴛ+[∂(VdP)/∂V]ᴛ=T(∂P∕∂T)ᵥ-P=T(R/V)-P=0
⸪PV=RT → PdV+VdP=0 ⸫-P=(VdP)/dV
(∂H∕∂P)ᴛ=T(∂S∕∂P)ᴛ+V=-T(∂V∕∂T)ₚ+V=-T(R/P)+V=0
or (∂U∕∂S)ᴛ=T+V(∂P∕∂S)ᴛ=T-V(∂T∕∂P)ᵥ=T-P (R/V)=0 所以H為T的函數
ratio relation z=z(x, y), (∂x∕∂y)z(∂y∕∂z)x(∂z∕∂x)y=-1
pf: dz=(∂z∕∂x)ydx+(∂z∕∂y)xdy → dz/[(∂z∕∂x)y]=dx+(∂z∕∂y)xdy/[(∂z∕∂x)y]=dx+(∂z∕∂y)x(∂x∕∂z)ydy ...(a)
x=x(y, z), dx=(∂x∕∂y)zdy+(∂x∕∂z)ydz ...(b), 比較(a),(b) → (∂x∕∂y)z=-(∂z∕∂y)x(∂x∕∂z)y
利用倒數關係, (∂x∕∂y)z(∂y∕∂z)x(∂z∕∂x)y=-1
Ex.-3 TdS equations
dS≡δQᵣₑᵥ/T → δQ=TdS → ∆Q=∫₁²δQ=∫₁²TdS
δQ=TdS=(?)dT+(?)dV 1st TdS equation→ TdS=f(T,V)
δQ=TdS=(?)dT+(?)dP 2nd TdS equation→ TdS=f(T,P)
δQ=TdS=(?)dP+(?)dV 3rd TdS equation→ TdS=f(P,V)
1st TdS equation S=S(T,V) → dS=(∂S∕∂T)ᵥdT+(∂S∕∂V)ᴛdV
TdS=δQ=cᵥdT i.e. cᵥ=(δQᵣₑᵥ/dT)ᵥ →cᵥ=T∙(∂S/∂T)ᵥ=(∂U/∂T)ᵥ ⸪dUᵛ=δQᵛ, cᵛ= δQᵥ/dT=(∂U/∂T)ᵥ
→dS=(cᵥ∕T)dT+(∂P∕∂T)ᵥdV→ dS=(cᵥ∕T)dT+(α∕β)dV
⸪P=P(T,V) its ratio relation, (∂P∕∂T)ᵥ(∂T∕∂V)ₚ(∂V∕∂P)ᴛ=-1
→ (∂P∕∂T)ᵥ=-(∂V∕∂T)ₚ/(∂V∕∂P)ᴛ=-αV/-βV=α/β
i.e. expansion coeff. α=(1/V)(∂V∕∂T)ₚ, compressibility β=(-1/V)(∂V∕∂P)ᴛ
1st TdS equation→ TdS=f(T,V)=cᵥdT+(αT∕β)dV
state 1(T₁,V₁) → state 2(T₂,V₂) ∆Q=∫₁²δQ=∫₁²TdS=∫ᴛ₁ᵀ²cᵥdT+∫ᴠ₁ᴠ²(αT∕β)dV
2nd TdS equation S=S(T,P) → dS=(∂S∕∂T)ₚdT+(∂S∕∂P)ᴛdP
δQₚ=TdSₚ=cₚdT , ⸪cₚ=(δQ/dT)ₚ ⸫(∂S∕∂T)ₚ=cₚ/T and (∂S∕∂P)ᴛ=-(∂V∕∂T)ₚ=-αV
dS=(∂S∕∂T)ₚdT+(∂S∕∂P)ᴛdP=(cₚ/T)dT-αVdP→TdS=cₚdT-αVTdP
Ex-4 Gibbs-Helmholtz equation: 定壓[d(∆G/T)/dT]ₚ=-∆H/T², 已知G(T) 如何計算H(T)
G≡H-TS →[∂(G/T)/∂T]ₚ=[∂(H/T)/∂T]ₚ-(∂S∕∂T)ₚ=[T(∂H/∂T)ₚ-H]/T²-cₚ/T=cₚ/T-H/T²-cₚ/T=-H/T²
i.e. df(y/x)=(xdy-ydx)/x²
同理[∂(G₂/T)/∂T]ₚ-[∂(G₁/T)/∂T]ₚ=-H₂/T²-(-H₁/T²) → [∂(∆G/T)/∂T]ₚ=-∆H/T²
experimental data: G vs. T plot transform to G/T vs. 1/T plot
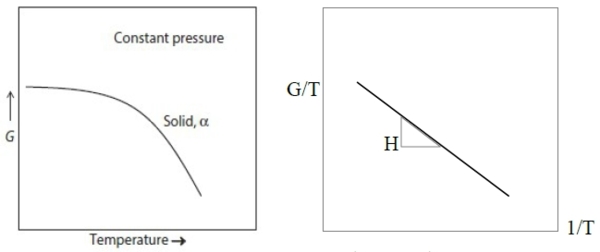
[∂(∆G/T)/∂T]ₚ=-∆H/T² → [∂(∆G/T)/∂(1/T)]ₚ=∆H
Ex-5 Helmholtz-internal energy equation: 定容[∂(∆A/T)/∂T]ᵥ=-∆U/T² 已知A(T) 如何計算U(T)
A≡U-TS, 同理[∂(A/T)/∂T]ᵥ=[∂(U/T)/∂T]ᵥ-(∂S∕∂T)ᵥ=[T(∂U/∂T)ᵥ-U]/T²-cᵥ/T=cᵥ/T-U/T²-cᵥ/T=-U/T²
Ex.-6 c≡δQ/dT , cₚ=(δQ/dT)ₚ, cᵥ=(δQ/dT)ᵥ
eq. 2-8, cₚ-cᵥ=(∂U∕∂V)ᴛ(∂V∕∂T)ₚ+P(∂V∕∂T)ₚ=(∂V∕∂T)ₚ[P+(∂U∕∂V)ᴛ]轉換成可量的物理量
⸪dA=-SdT-PdV ⸫(∂A/∂V)ᴛ=-P, and A≡U-TS (∂A/∂V)ᴛ=(∂U/∂V)ᴛ-T(∂S/∂V)ᴛ=(∂U/∂V)ᴛ-T(∂P/∂T)ᵥ
→ -P=(∂U/∂V)ᴛ-T(∂P/∂T)ᵥ代入eq. 2-8, cₚ-cᵥ=T(∂V∕∂T)ₚ(∂P/∂T)ᵥ
⸪(∂P∕∂T)ᵥ(∂T∕∂V)ₚ(∂V∕∂P)ᴛ=-1 → (∂P∕∂T)ᵥ=-(∂V∕∂T)ₚ/(∂V∕∂P)ᴛ=-αV/-βV=α/β
⸫cₚ-cᵥ=T(∂V∕∂T)ₚ(∂P/∂T)ᵥ=-T(∂V∕∂T)²ₚ/(∂V∕∂P)ᴛ=α²VT/β
i.e. expansion coeff. α=(1/V)(∂V∕∂T)ₚ, compressibility β=-(1/V)(∂V∕∂P)ᴛ
Ex. Al=26.98 g/mole, 求cᵥ=? (cₚ=24.36 J/Kmole, T=298K, α=7.3510⁻⁵ /K, β=1.210⁻⁶ /atm, ρ=2.7 g/cm³)
V=26.98/2.7≈10 cm³/mole=10⁻² l/mole, cᵥ=cₚ-(α²VT/β)=23.13 J/Kmole
Ex. adiabatic thermoelastic effect: 利用施加突然的壓力轉成熱使溫度升高,做非破壞的缺陷檢測
Al₂O₃ 施加sudden ∆P=500 MPa, 求∆T=? (Given: cₚ=80 J/Kmole, T=298K, α=2.210⁻⁵ /K, V=25.6 cm³/mole)
⸪sudden loading ∆P and no heat dissipation→adiabatic and heat make T increase
⸫(∂T/∂P)ₛ=? (∂T∕∂P)ₛ(∂P∕∂S)ᴛ(∂S∕∂T)ₚ=-1 and cᵥ=T∙(∂S/∂T)ᵥ, cₚ=T(δS/dT)ₚ=(δH/dT)ₚ
(∂S∕∂P)ᴛ=-(∂V∕∂T)ₚ=-αV → (∂T/∂P)ₛ=-(∂S∕∂P)ᴛ/(∂S∕∂T)ₚ= αV/(cₚ/T)=αVT/cₚ
→ ∫ᴛ₁ᵀ²dT/T=∫ᴩ₁ᴾ₂(αV/cₚ)dP=αV/cₚ(P₂-P₁), T₂=299K, ∆T=1K
補充: Dehoff ch-4, pp.51-71 general strategy for deriving thermodynamic equations
e.g. 5-11 (∂T/∂P)ₛ=? 2nd TdS equation TdS=cₚdT-αVTdP ⸪dS=0 ⸫(∂T/∂P)ₛ=αVT/cₚ
概念1: 溫度和壓力是實作上最容易操作的變數,自變數以T, P表示,其他變數(V, S, U, H, A, G)都轉換成以自變數T, P表示的函數Z=Z(T, P) → dZ=(?)dT+(?)dP
V=V(T,P) → dV=(∂V∕∂T)ₚdT+(∂V∕∂P)ᴛ dP=αVdT- βVdP
S=S(T,P) → dS=(∂S∕∂T)ₚdT+(∂S∕∂P)ᴛdP=(cₚ/T)dT-αVdP i.e. (∂S∕∂P)ᴛ=-(∂V∕∂T)ₚ=-αV
dU=TdS- PdV=T[(cₚ/T)dT-αVdP]-P[αVdT- βVdP]=(cₚ-αPV)dT+V(βP-αT)dP
dH=TdS+VdP=T[(cₚ/T)dT-αVdP]+VdP=cₚdT+V(1-αT)dP
dA=-SdT-PdV=-SdT-P(αVdT- βVdP)=-(S+αPV)dT+βPVdP
dG=-SdT+VdP
基礎公式:
dV=αVdT- βVdP, dS=(cₚ/T)dT-αVdP, dU=(cₚ-αPV)dT+V(βP-αT)dP,
dH=cₚdT+V(1-αT)dP, dA=-(S+αPV)dT+βPVdP, dG=-SdT+VdP
概念2:Z=Z(x,y), dZ=Mdx+Ndy 將dx=XᴛdT+XₚdP, dy=YᴛdT+YₚdP代入dZ
dZ=M(XᴛdT+XₚdP)+N(YᴛdT+YₚdP)=(MXᴛ+NYᴛ)dT+(MXₚ+NYₚ)dP
再比較dZ=ZᴛdT+ZₚdP解M, N
習題練習:
5-1, 5-2, 5-9 S=S(P,V) dS=MdP+NdV=MdP+N(αVdT-βVdP)= NαVdT+(M-NβV)dP
與dS=(cₚ/T)dT-αVdP 比較, NαV=cₚ/T, M-NβV=-αV ⸫M=βcₚ/αT- αV, N=cₚ/αVT,
(∂S∕∂P)ᵥ=M=βcₚ/αT- αV=βcᵥ/αT, (∂S∕∂V)ₚ=N=cₚ/αVT, i.e. cₚ-cᵥ=α²VT/β
(∂P∕∂V)ₛ=-N/M=-(cₚ/αVT)/(βcᵥ/αT)=-cₚ/βcᵥV
3rd TdS equation: S=S(P,V) TdS=(cₚ/αV)dV+(βcᵥ/α)dP
5-3, 5-4 A=A(P,V) dA=MdP+NdV=MdP+N(αVdT-βVdP)= NαVdT+(M-NβV)dP
與dA=-(S+αPV)dT+βPVdP 比較, NαV=-(S+PVα), M-NβV=PVβ
⸫M=-βS/α, N=-(S+PVα)/αV, (∂A∕∂P)ᵥ=M=-βS/α, (∂A∕∂V)ₚ=N=-(S+PVα)/αV
dA=(-βS/α)dP-[(S+PVα)/αV]dV
5-5, 5-6 H=H(S,V) dH=MdS+NdV=M(cₚ/TdT-VαdP)+N(αVdT-βVdP)=(Mcₚ/T+NαV)dT-(MVα+βNV)dP, 與dH=cₚdT+V(1-αT)dP比較, Mcₚ/T+NαV=cₚ, Mα+βN=αT-1
⸫M=T(1+αV/βcᵥ), N=-cₚ/βcᵥ, (∂H∕∂S)ᵥ=M=T(1+αV/βcᵥ), (∂H∕∂V)ₛ=N=-cₚ/βcᵥ
dH=T(1+αV/βcᵥ)dS-(cₚ/βcᵥ)dV
5-8 S=S(T,P) → dS=(∂S∕∂T)ₚdT+(∂S∕∂P)ᴛdP=(cₚ/T)dT-αVdP i.e. (∂S∕∂P)ᴛ=-(∂V∕∂T)ₚ=-αV
⸪dS=0 ⸫(∂T/∂P)ₛ=αVT/cₚ
5-10. dG=-SdT+VdP→ (∂G∕∂P)ᴛ=V → [∂(∂G∕∂P)ᴛ/∂P]ᴛ=(∂V∕∂P)ᴛ
dA=-SdT-PdV→ (∂A∕∂V)ᴛ=-P → [∂(∂A∕∂V)ᴛ/∂V]ᴛ=-(∂P∕∂V)ᴛ ⸫(∂²G∕∂P²)ᴛ=-1/(∂²A∕∂V²)ᴛ
5-7.
(∂cₚ∕∂P)ᴛ=[∂(∂H∕∂T)ₚ∕∂P]ᴛ=[∂(∂H∕∂P)ᴛ∕∂T]ₚ=[∂(V-αVT)∕∂T]ₚ=(∂V∕∂T)ₚ-[αV+αT(∂V∕∂T)ₚ+VT(∂α∕∂T)ₚ]
i.e. dV=αVdT- βVdP, dH=cₚdT+V(1-αT)dP
→ (∂cₚ∕∂P)ᴛ=(∂V∕∂T)ₚ-[αV+αT(∂V∕∂T)ₚ+VT(∂α∕∂T)ₚ]=-[α²+(∂α∕∂T)ₚ]VT
application to monatomic ideal gas, δQ=TdS → ∆Q=∫₁²δQ=∫₁²TdS
p.s. cᵥ=3R/2, cₚ=5R/2, γ=5/3, cₚ-cᵥ=R, α=1/T, β=1/P,
U(T) and H(T)與P,V無關, dU=cᵥdT, dH=cₚdT
DeHoff Ex.4-7 1 mole monatomic ideal gas changes from state 1(T₁=298K, P₁=1 atm) to state 2(T₂=298K, P₂=1000 atm), ask ∆S=?
Sol: S=S(T,P) → dS=(∂S∕∂T)ₚdT+(∂S∕∂P)ᴛdP=(cₚ/T)dT-αVdP
∆S=∫₁²dS=∫ᴩ₁ᴾ₂-αVdP=-∫ᴩ₁ᴾ₂(V/T)dP=-∫ᴩ₁ᴾ₂(R/P)dP=Rln(P₁/P₂)=-57.4 J/K
Ex.4-10 Ask ∆Q=? by following process. 1. reversible isothermal P₁(5 atm) → P₂(1 atm). 2. reversible isobaric V₁ → V₂. 3. reversible isochoric T₁ → T₂.
Sol: 1. S=S(T,P) ⸫TdS=cₚdT-αVTdP →∆Qᴛ =∫₁²TdS=∫ᴩ₁ᴾ₂-αTVdP=-∫ᴩ₁ᴾ₂(RT/P)dP=RTln(P₁/P₂)
2. S=S(P,V) ⸫TdS=(cₚ/αV)dV+(βcᵥ/α)dP→∆Qₚ=∫₁²TdS=∫ᴠ₁ᴠ²(cₚ/αV)dV=∫ᴠ₁ᴠ²(cₚT/V)dV= cₚTln(V₂/V₁)=5RTln(V₂/V₁)/2
3. S=S(T,V) ⸫TdS=f(T,V)=cᵥdT+(αT∕β)dV→∆Qᵥ=∫₁²TdS=∫ᴛ₁ᵀ²cᵥdT=cᵥ(T₂-T₁)
Ex.4-11 1 mole monatomic ideal gas changes from state 1(T₁=273K, P₁=1 atm) to state 2(T₂=?K, P₂=0.5 atm) by the change of V=22.4P , ask ∆Q=? T₂=?
Sol: ideal gas follow PV=RT, V=22.4P=RT/P → RT=22.4P² ⸫T₂= 22.4P₂²/R=68.2K
∆Q=∫₁²TdS=∫ᴛ₁ᵀ²cₚdT -∫ᴩ₁ᴾ₂αTVdP=cₚ(T₂-T₁)-∫ᴩ₁ᴾ₂22.4PdP=cₚ(T₂-T₁)-11.2(P₂²-P₁²)







